Thrombolex is committed to helping provide a new standard of care for the treatment of arterial and venous thromboembolic (A+VTE) conditions. Discover our innovative approach to treatment with the BASHIR™ Family of Endovascular Catheters.
An Elegant, Novel Solution
For Optimal Thrombus Resolution
The Problem
Treating A+VTE safely and effectively while minimizing risk, maximizing clot burden resolution, and producing consistent clinical outcomes is of critical importance.
Our Solution
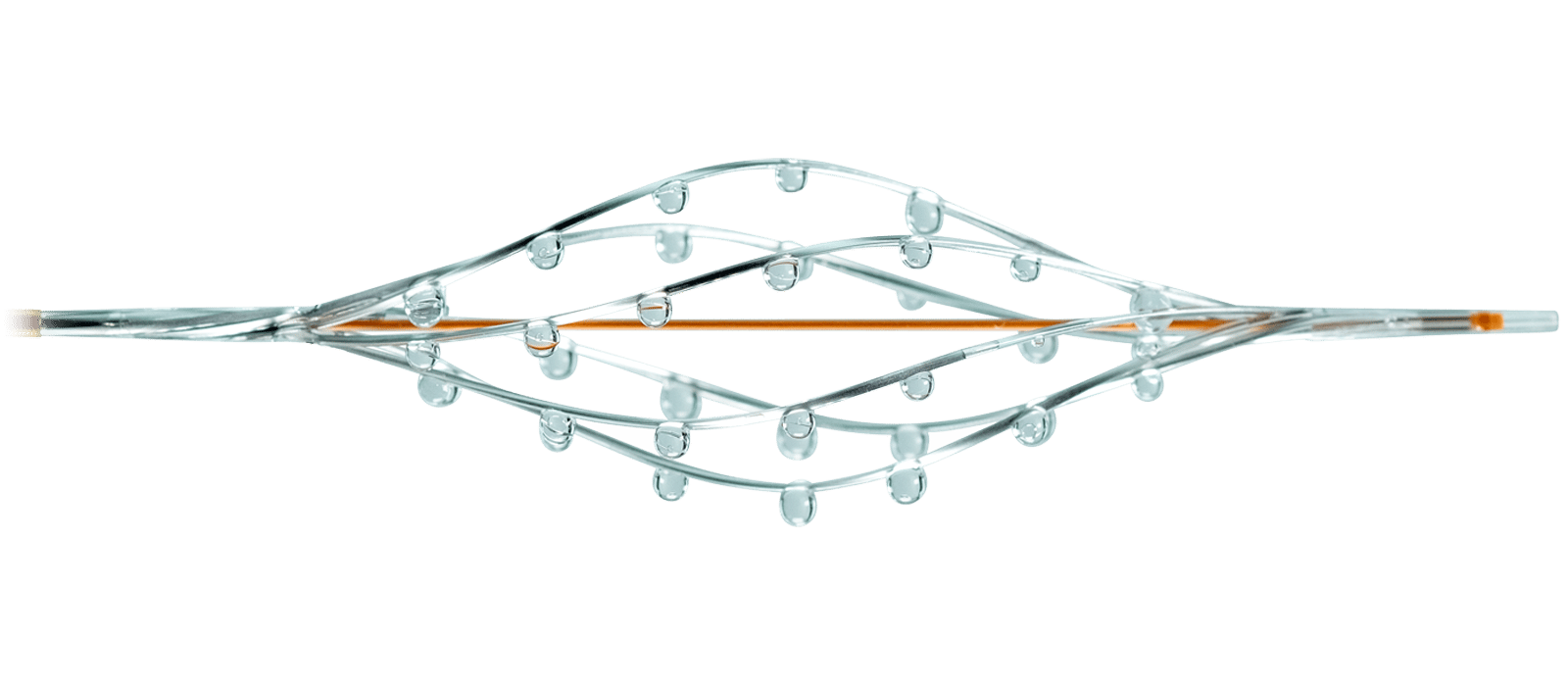
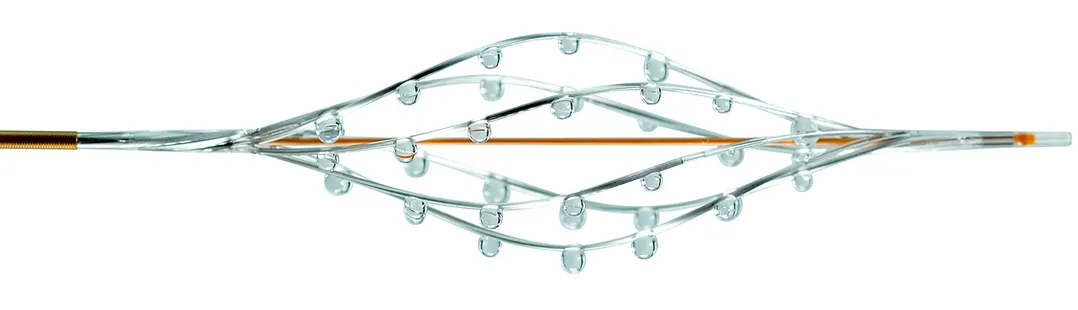
The unique hybrid mechanism of action of the BASHIR™ family of endovascular catheters allows the use of both mechanical and pharmacological treatment to immediately restore blood flow. This allows for the efficient and safe introduction of thrombolytics to produce positive clinical outcomes.
Product Resources
Product Overview • Product Details
Clinical Resources
Clinical Studies • Instructional Videos
Our Company
Our Mission • Our Leadership
Our Family of Catheters
For restoring blood flow in peripheral and pulmonary vessels
The catheter’s expandable infusion basket allows a controlled and selective infusion of physician-specified fluids.
Available in 7F and 8F sizes.
For restoring blood flow within shorter peripheral and pulmonary vessels
The short basket of the S-B catheter allows navigation and implementation in shorter, more tortuous vessels.
Avialable in 7F and 8F sizes.
For restoring blood flow in peripheral vessels with extensive thrombus burden
A family of catheters designed to deliver thrombolytics through the expandable infusion basket and along a segment of the catheter shaft.
–





Experience the ease and efficiency of our BASHIR™ Endovascular Catheters, delivering clinically proven efficacy and safety when treating acute PE. Discover more at Thrombolex.com #thrombolex ... See MoreSee Less
0 CommentsComment on Facebook
Optimize your approach to treating PE using the BASHIR™ .035 Endovascular Catheter with 0.035" guidewire compatibility. Recent RESCUE II Study outcomes showed remarkable on-the-table efficacy with improved RV/LV ratio and hemodynamics. Less time. Lower dose. Better Outcomes. Thrombolex.com #Thrombolex ... See MoreSee Less
0 CommentsComment on Facebook
Transform your PERT program with the BASHIR™ Endovascular Catheter. Our novel technology streamlines intervention, enabling improved clinical outcomes. Discover our pioneering PE protocol to enhance patient care at Thrombolex.com #thrombolex ... See MoreSee Less
0 CommentsComment on Facebook
Experience the Thrombolex difference with our BASHIR™ .035 Endovascular Catheter. Compelling initial on-the-table results from the RESCUE II study showed positive improvement in RV/LV ratio after 48 hours and a significant improvement in hemodynamics while on-table. The total time of the procedure was 47 minutes, with lysis only taking 27 minutes with no thrombolytic infusion post-procedure. The patient recovered on room air and without an ICU stay. Visit Thrombolex.com #thrombolex ... See MoreSee Less
0 CommentsComment on Facebook
Thank you to SCAI for another enlightening and informative conference. To learn more about how the innovative BASHIR™ Family of Endovascular Catheters can elevate your PERT program, visit Thrombolex.com #thrombolex #scai2024 ... See MoreSee Less
0 CommentsComment on Facebook
Join us at SCAI today to hear about how the BASHIR™ Endovascular Catheter has been clinically proven to effectively and safely treat acute PE by pharmacomechanical lysis (PML) through the compelling results of the NIH-funded IDE RESCUE Trial. Be sure to ask about a demo of our 0.035" guidewire compatible BASHIR™ .035 and BASHIR™ S-B .035 Endovascular Catheters, and learn how our PE protocol optimizes PE procedure time. Thrombolex.com #Thrombolex #SCAI2024 ... See MoreSee Less
0 CommentsComment on Facebook